Effect of acidification on micro structure and adsorption characteristics of coal
-
摘要:
为探究酸化作用下煤体微观结构的改变及其对煤吸附特性的影响机理,对氢氟酸处理前后的煤样开展了低温氮气吸附实验、红外光谱实验及等温吸附实验,分析了酸液对煤体孔隙结构及分子结构的影响,并探究了酸化作用下煤体吸附特性的演变机制。结果表明:酸处理后,煤样的平均孔径、总孔体积增大,而比表面积降低,酸液对煤体具有扩孔增容的作用;酸化作用可以改变煤体的微观分子结构,酸化后煤样的苯环结构逐渐向多取代苯环发展,较长的脂肪链及结构较弱的氢键结构被破坏,同时含氧官能团的相对含量大幅增加;等温吸附实验中,酸化后煤样对甲烷的极限吸附量和吸附速率分别下降14.02%和23.58%;吸附势理论表明酸化后煤样对甲烷吸附能力降低、甲烷的吸附空间减少。
Abstract:To investigate the changes in the microstructure of coal under acidification and the mechanism of their impact on coal adsorption characteristics, low-temperature nitrogen adsorption experiments, infrared spectroscopy experiments, and isothermal adsorption experiments were conducted on coal samples before and after hydrofluoric acid treatment. The influence of acid solution on the pore structure and molecular structure of coal was analyzed, and the evolution mechanism of coal adsorption characteristics under acidification was explored based on the experimental results. The results showed that after acid treatment, the average pore size and total pore volume of the coal sample increased, while the specific surface area decreased. The acid solution has the effect of expanding pores and increasing capacity of pores. Acidification can change the micro molecularstructure of coal. After acidification, the benzene ring structure of coal samples gradually developed towards multi substituted benzene rings, and the longer fatty chains and weaker hydrogen bond structures were destroyed. Besides, the content of oxygen-containing functional groups increased. In the isothermal adsorption experiment, the maximum adsorption capacity and adsorption rate of methane on coal samples decreased by 14.02% and 23.58% respectively after acidification. Furthermore,the adsorption potential theory indicated that the adsorption capacity of coal samples for methane decreased and the adsorption space for methane decreases after acidification.
-
煤层气是指储存在煤层中以甲烷为主的一种烃类气体,其在煤储层中主要以吸附态的形式存在,故煤层对甲烷吸附性能的优劣将直接影响煤层气的抽采效率[1-2]。酸化技术是强化煤层气抽采的重要措施之一,目前国内外已开展了一系列煤层酸化增透的室内实验和现场应用。苏现波等[3]发现酸液能有效溶蚀堵塞煤层的矿物、改善煤层气的导流通道。WANG等[4]研究了酸化前后煤样孔隙结构的变化,结果表明:酸化后煤样中微孔的孔体积下降,中孔和大孔的数量及孔体积明显增加。马永元等[5]测定了酸化前后煤样孔隙结构和吸附能力的变化,发现酸处理能改变煤样的孔隙组成,使煤样的吸附孔数量减少、吸附能力降低。原文杰[6]测定了不同酸化条件下煤样孔隙结构和吸附特性的变化,发现在适当的酸化条件下煤样的微孔及过渡孔具有向中、大孔转化的趋势,且酸化后煤样对甲烷吸附能力降低。
随着研究的深入,一些学者逐渐把目光聚焦在了煤的微观分子结构上,探索酸液对煤体分子结构的影响。王芳芳等[7]使用盐酸对煤样进行了酸化处理,发现酸化后煤样的富氢指数明显减小而芳构化指数和富氧指数明显增大。贺成杰等[8]使用盐酸与氢氟酸依次对煤进行处理,发现酸化后煤样的苯环取代方式发生改变、含氧官能团的含量增加。张锐等[9]使用氢氟酸对煤样进行处理,发现酸化后煤样中含氧官能团的含量上升,煤样的润湿性增强。
上述研究从微细观结构的变化剖析了酸液对煤体理化结构的影响,并通过孔隙结构的变化探究了酸液对煤体吸附特性的影响机理,但尚未进一步揭示酸化作用下微细观结构相互转化的内在机制及其对煤体吸附能力的影响。因此,本文以贵州矿区无烟煤为研究对象,使用氢氟酸处理煤样,拟通过低温氮气吸附实验、红外光谱实验(FTIR)及等温吸附实验探究酸化前后煤样微观结构和吸附能力的变化,并借助吸附势理论分析酸化作用下煤样吸附特性的演化规律,从而深入理解酸液对煤体吸附特性的影响机制。
1. 实 验
实验所用无烟煤取自贵州省金沙县林华煤矿M9煤层的1091掘进面,取样点埋深约296 m,标高+787 m。在开采面的新鲜暴露处取得所需煤块后,即用保鲜膜包裹送至实验室加工。经过破碎筛分后,选取粒径在0.18~0.25 mm范围的煤样,进行低温氮气吸附实验和等温吸附实验,并挑选粒径小于0.075 mm的煤样用于FTIR测试。
1)酸化实验。对实验煤样开展了X射线衍射实验并对谱图进行物相检索,结果表明:煤样中硅酸盐矿物含量较高,约占总矿物组分的91.60%,而碳酸盐矿物含量较低,仅占4.80%。张小东等[10]研究发现,HF可溶蚀煤中大部分矿物,酸化溶蚀效果显著,故考虑使用HF对煤样进行酸化处理。使用去离子水将质量分数为40%的HF稀释至6%,随后将煤样浸泡于6%的HF溶液中12 h,反应完毕后过滤并转入干燥箱中,在85℃的条件下干燥至恒重,处理完毕后放入试样袋中密封备用。
2)低温氮气吸附实验。实验采用3H-2000PS型静态容量法比表面积及孔结构分析仪进行低温氮气吸附实验。将煤样在90℃的条件下抽真空预处理4 h,去除孔内吸附杂质,然后用纯度大于99.99%的氮气作为吸附质进行实验,得到吸附/脱附曲线。根据实验结果,分别利用BET、BJH理论模型计算煤样的比表面积、平均孔径等孔隙结构参数。
3)FTIR实验。FTIR实验采用Nicolet 6700型傅里叶变换红外光谱仪测定,扫描范围为400~4000 cm−1,分辨率为4 cm−1,每次测定共扫描32次。取少量煤粉与溴化钾按1:100的比例充分混合研磨,将混合物在10 MPa的压力下压制30 s,成片后放入仪器进行扫描。
4)等温吸附实验。实验采用HCA-1型高压容量法瓦斯吸附装置进行等温吸附实验,实验温度为30℃,吸附质为纯度大于99.99%的甲烷气体。在吸附实验中设定6个平衡压力点,当监测到甲烷压力值在0.5 h内变化不超过0.01 MPa时,即认为达到了吸附平衡。
2. 实验结果
2.1 低温氮气吸附
酸化前后煤样吸附/脱附曲线如图1,图中p为氮气平衡压力;p0为氮气饱和蒸汽压力。由图1可知,酸化后煤样的吸附/脱附曲线的回滞现象明显小于酸化前煤样,说明经过酸处理后煤样的孔隙连通性得到改善。同时,相较于酸化前,酸化后煤样对液氮的吸附量显著降低,降幅约为36.35%。
低温氮气吸附实验可以表征煤样的孔隙发育情况,运用BET和BJH理论模型分析得到实验煤样的孔隙结构参数,酸化前后煤样孔隙结构特征见表1。
表 1 酸化前后煤样孔隙结构特征Table 1. Pore structure characteristics of coal samples before and after acidfication煤样 平均孔径/
nm比表面积/
(m2·g−1)孔体积/
(cm3·g−1)最可几孔径/
nm酸化前 2.64 45.0614 0.0026 1.48 酸化后 8.97 8.4283 0.0096 28.09 由表1可知,酸化后煤样的平均孔径、孔体积、最可几孔直径均有不同幅度的增大,分别为酸化前煤样对应值的3.40、3.69、18.98倍,而比表面积下降了81.96%。宋申等[11]指出最可几孔径代表煤中出现概率最大的孔径,可以反映孔径的分布情况。同时,戚灵灵等[12]研究发现煤样的比表面积主要由微、小孔贡献,其数量越多则比表面积越大、甲烷吸附空间越大。结合表1可以发现,酸化后煤样的平均孔径、最可几孔径、总孔体积增大而比表面积降低,说明酸化后煤样中的矿物被溶蚀,微、小孔数量大幅减少,煤样出现了显著的扩孔增容现象。
2.2 FTIR分析
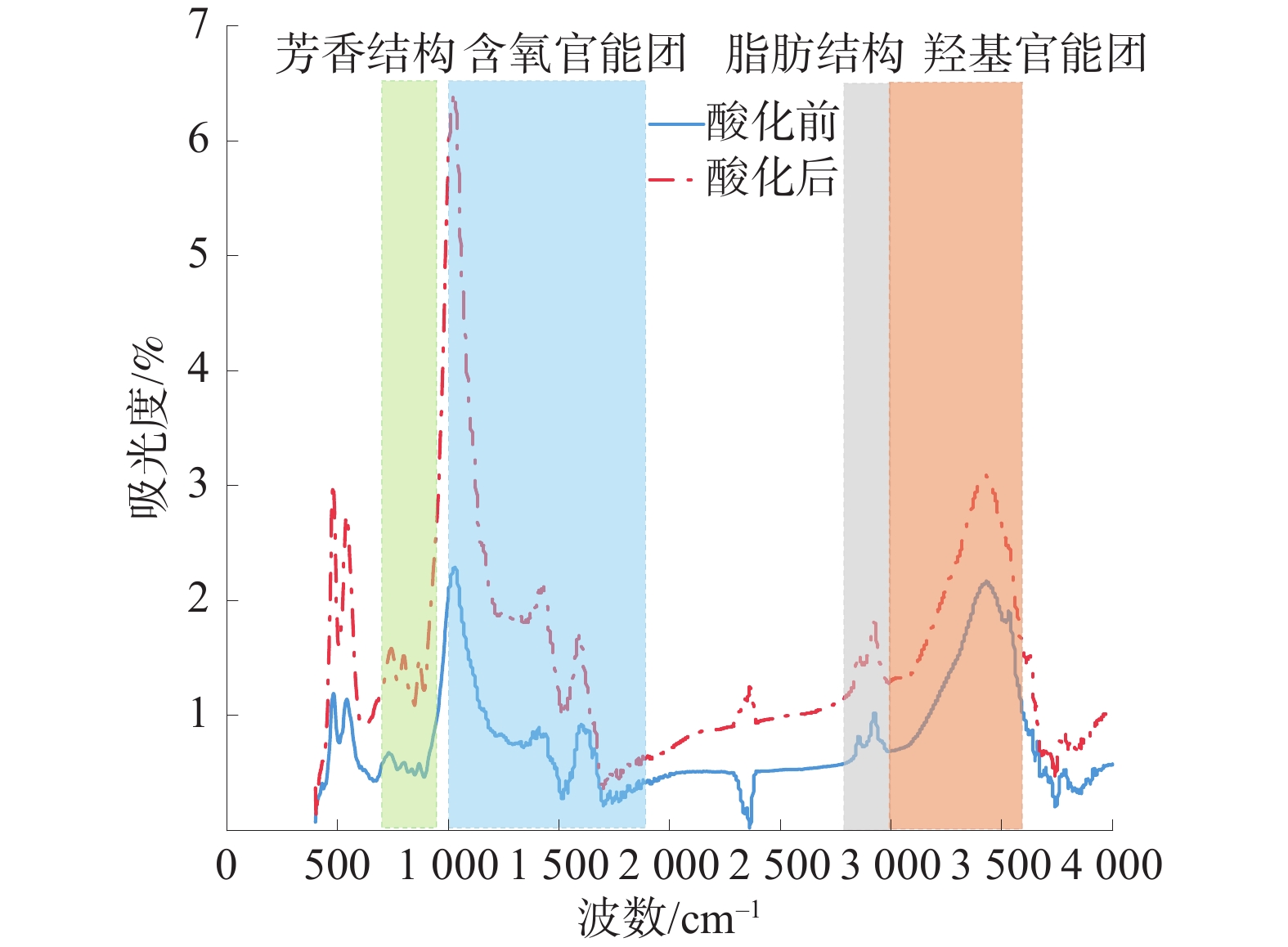
研究表明,煤的微观分子结构对其甲烷吸附、解吸能力有显著影响,烷基侧链能促进煤对甲烷的吸附而含氧官能团会抑制其对甲烷的吸附[13-14]。酸化前后煤样红外光谱谱图如图2。
由图2可知,酸化后煤样在含氧官能团和羟基官能团及芳香结构波段上的吸收峰强度较酸化前大幅增加,说明在酸性条件下煤中官能团会积极参加氧化反应,从而改变煤中官能团的含量及分布。考虑某一波段的吸收峰可能是由多个吸收峰叠加而成,故需要对谱图进行分峰拟合,以深入分析酸化处理对煤分子结构的影响。根据官能团归属情况,将实验煤样的红外光谱谱图分为4部分:芳香结构、含氧官能团、脂肪结构及羟基结构,并依次对各波段进行分峰拟合[15]。
2.2.1 芳香结构分峰拟合
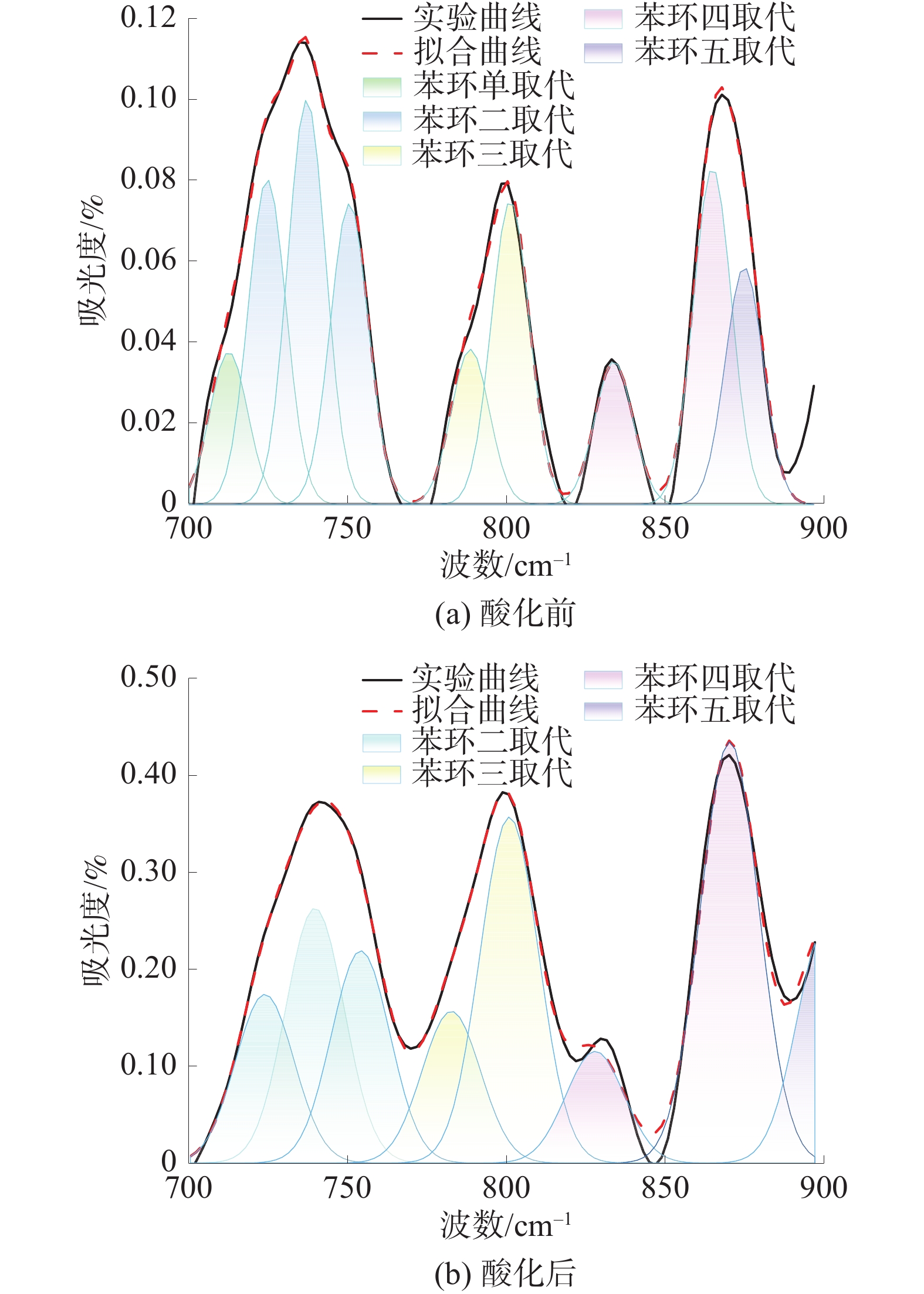
煤中芳香结构的特征吸收峰主要集中在700~900cm−1波段,酸化前后煤样芳香结构分峰拟合结果图3。
由图3可知,酸化后苯环单取代对应的吸收峰消失,苯环二取代所占比例下降,而苯环三取代、四取代及五取代所占比例上升,说明在酸液作用下煤中芳香结构发生了取代反应,苯环单取代和二取代逐渐向多取代转变,使得苯环上的氢原子数量减少、侧链数量增多。
2.2.2 含氧官能团分峰拟合
煤中含氧官能团的特征吸收峰主要集中在1000~1800 cm−1波段,酸化前后煤样含氧官能团分峰拟合结果如图4。研究表明,含氧官能团对煤样吸附特性影响较大:一方面,含氧官能团会占据吸附位点、减少甲烷的吸附空间[16];另一方面,含氧官能团的亲水性较强,会加剧煤中的气水竞争吸附[17]。由图4可知,酸化后煤样中的含氧官能团的种类和占比大幅增加,说明酸化过程中伴随着含氧官能团的生成与转化且酸化后煤样对甲烷的吸附能力降低。同时,XING等[18]通过分子模拟发现含氧官能团脱除后,煤晶胞中的总孔体积增加,故推测酸化作用下煤中官能团发生破坏、转化会导致微、小孔的孔径增大,从而导致实验煤样的比表面积降低、甲烷的吸附空间减少。
2.2.3 脂肪结构的谱峰拟合
煤中脂肪结构的特征吸收峰主要集中在2800~3000 cm−1波段,酸化前后煤样脂肪结构分峰拟合结果见表2。
表 2 酸化前后煤样脂肪结构分峰拟合结果Table 2. Peak fitting results of coal sample fat structure before and after acidification煤样 波数/cm−1 归属 面积占比/% 酸化前 2826.46 -CH2对称振动 1.26 2853.83 -CH2对称振动 20.19 2878.85 -CH3对称振动 8.86 2900.12 -CH不对称振动 12.85 2921.84 -CH2不对称振动 34.53 2937.38 -CH3不对称振动 10.24 2959.58 -CH3不对称振动 12.06 酸化后 2827.13 -CH2对称振动 3.08 2853.20 -CH2对称振动 20.27 2877.47 -CH3对称振动 11.24 2899.00 -CH不对称振动 15.47 2921.01 -CH2不对称振动 30.05 2935.85 -CH3不对称振动 9.68 2958.18 -CH3不对称振动 10.21 表2中各脂肪结构含量变化幅度各异,为进一步探究酸化作用下脂肪结构的变化,引入脂肪结构参数$ {\mathrm{I}}_{4} $来表征脂肪链长度,其值越大则脂肪链长度越长,计算公式如下:
$$ {\mathrm{I}}_{4}=\frac{{\mathrm{A}}_{\left({\mathrm{C}\mathrm{H}}_{2}\right)}}{{\mathrm{A}}_{\left({\mathrm{C}\mathrm{H}}_{3}\right)}} $$ (1) 式中:$ {\mathrm{A}}_{\left({\mathrm{C}\mathrm{H}}_{2}\right)} $为2920~3000 cm−1波段的-CH2含量,%;$ {\mathrm{A}}_{\left({\mathrm{C}\mathrm{H}}_{3}\right)} $为2920~3000 cm−1波段的-CH3含量,%。
酸化前后,煤样脂肪结构参数$ {\mathrm{I}}_{4} $的值分别为1.55、1.51,酸化后下降了2.43%。表明酸化后煤样的脂肪链长度缩短、支链化程度增加。结合图3、图4进行分析,认为酸化作用下连接在苯环上较长的脂肪链发生了破坏重组,生成了数量更多的含氧官能团及较短的侧链。
2.2.4 羟基结构的谱峰拟合
煤中羟基结构的特征吸收峰主要集中在3000~3600 cm−1波段,酸化前后煤样羟基结构分峰拟合结果见表3。
表 3 酸化前后煤样羟基结构分峰拟合结果Table 3. Fitting results of hydroxyl structure peaks in coal samples before and after acidification煤样 波数/cm−1 归属 面积占比/% 酸化前 3197.32 环氢键 49.68 3320.74 羟基醚氢键 21.27 3439.68 自缔合羟基氢键 19.27 3540.19 羟基$ \mathrm{\pi } $氢键 9.78 酸化后 3179.24 环氢键 36.40 3414.54 自缔合羟基氢键 53.55 3512.85 羟基π氢键 10.05 由表3可知,酸化后煤中环氢键、羟基π氢键的含量均有较大幅度的减小、羟基醚氢键甚至完全消失,而自缔合羟基氢键的含量增加至酸化前对应值的2.52倍。上述3种氢键在酸性环境下不稳定、易被破坏,且酸化处理会促进部分羟基结构的生成。
2.3 等温吸附实验
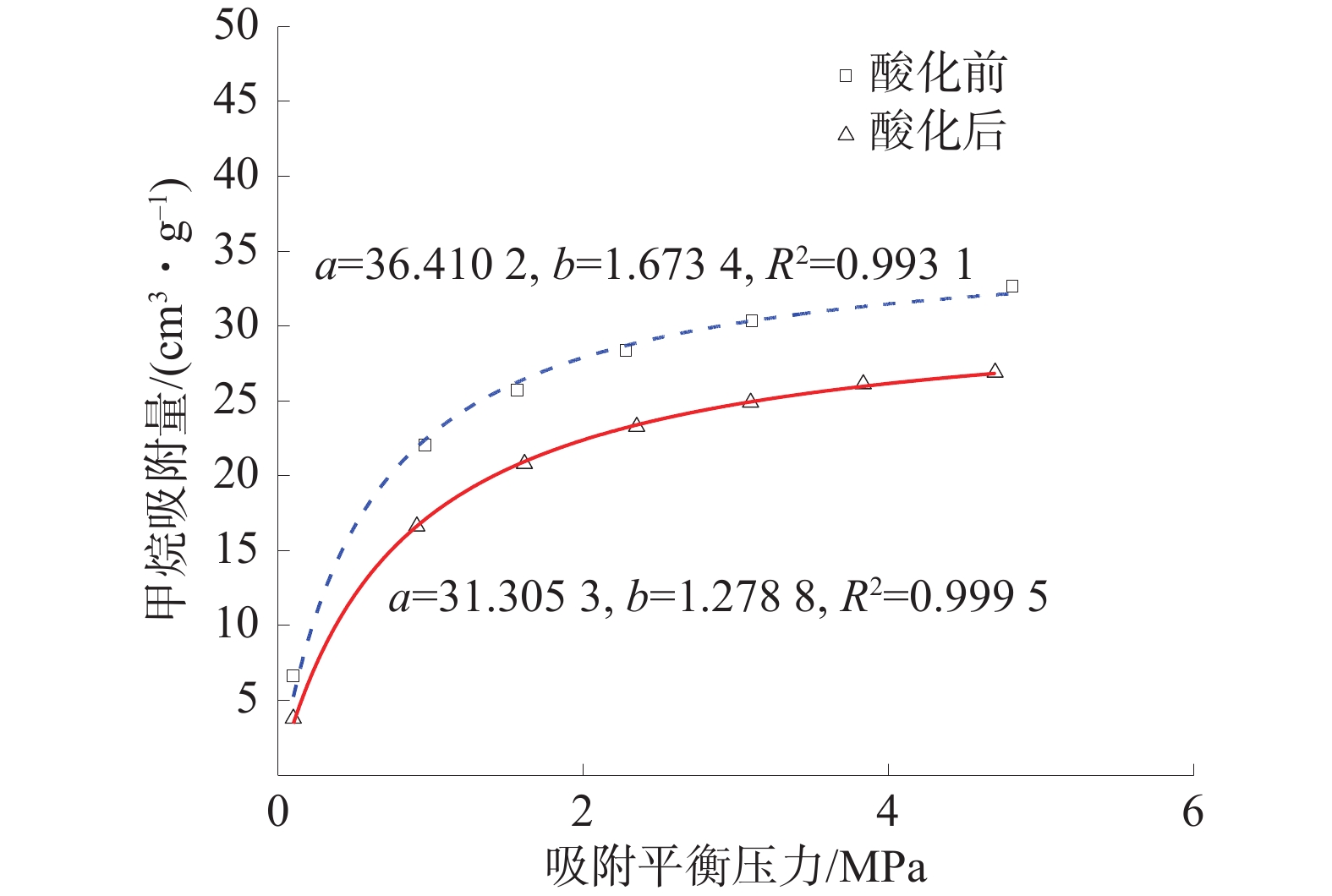
将等温吸附实验所得实验数据进行langmuir拟合,可得酸化前后实验煤样的等温吸附曲线及吸附常数a、b,酸化前后煤样的等温吸附曲线如图5。
由图5可知,实验煤样的甲烷吸附量随压力增大而增多。但随着压力不断增大,煤样的吸附速率在气压约1 MPa处骤降,并不断放缓直至吸附平衡。相较于酸化前,酸化后煤样的a、b值分别下降了14.02%和23.58%,说明酸化后煤样对甲烷的吸附量及吸附速率下降,酸液抑制了煤样的吸附能力。
2.3 吸附势特性曲线
吸附势理论是Polanyi于1914年提出的适用于物理吸附的热力学理论,可以反映物体吸附1mol吸附质所需要做的功[19]。以吸附势理论中的吸附势为纵轴、吸附相体积为横轴,即可得到吸附势特性曲线,且同一试样在不同温度下所得曲线唯一。运用吸附势理论来研究酸化前后煤样吸附特性的变化规律,可以预测某一压力条件下煤样的吸附量,进而为煤层酸化改造及煤层气抽采提供一定参考。
由吸附势理论可知,吸附势与压力和温度的表达式如下[20]:
$$ \varepsilon ={\int }_{{P}_{i}}^{{P}_{0}}\frac{RT}{P}dP=RT\mathrm{ln}\frac{{P}_{0}}{{P}_{i}} $$ (2) 式中:$ \varepsilon $为吸附势,J/mol;$ {p}_{0} $为气体饱和蒸汽压力,MPa;$ {p}_{i} $为气体吸附时的平衡压力,MPa;R为常数,取8.314 J/(mol·k);T为吸附平衡温度,取303.15 K。
由于甲烷在煤体表面吸附时的温度处于临界温度之上,故采用Dubinin建立的超临界条件下的虚拟饱和蒸汽压力的经验计算公式代替[21],即:
$$ {p}_{0}={p}_{\mathrm{c}}{\left(\frac{T}{{T}_{c}}\right)}^{2.7} $$ (3) 式中:$ {p}_{\mathrm{c}} $为甲烷的临界压力,取4.62 MPa;$ {T}_{\mathrm{c}} $为甲烷的临界温度,取190.6 K。
吸附相体积代表了气体在煤中占据的空间,计算表达式如下[22]:
$$ \omega =\frac{{M}_{g}{V}_{g}}{22\;400{\rho }_{ad}} $$ (4) 式中:$ \omega $为吸附相体积,cm3/g;$ {M}_{g} $为甲烷的摩尔质量,g/mol;$ {V}_{g} $为平衡条件下的气体吸附量,cm3/g;$ {\rho }_{ad} $为气体吸附相密度,g/cm3。
其中,气体吸附相密度$ {\rho }_{ad} $可由经验公式得[23]:
$$ {\rho }_{ad}={\rho }_{b}\mathrm{e}\mathrm{x}\mathrm{p}[-0.002\;5(T-{T}_{c}\left)\right] $$ (5) 式中:$ {\rho }_{b} $为沸点下甲烷的密度,取0.424 g/cm3。
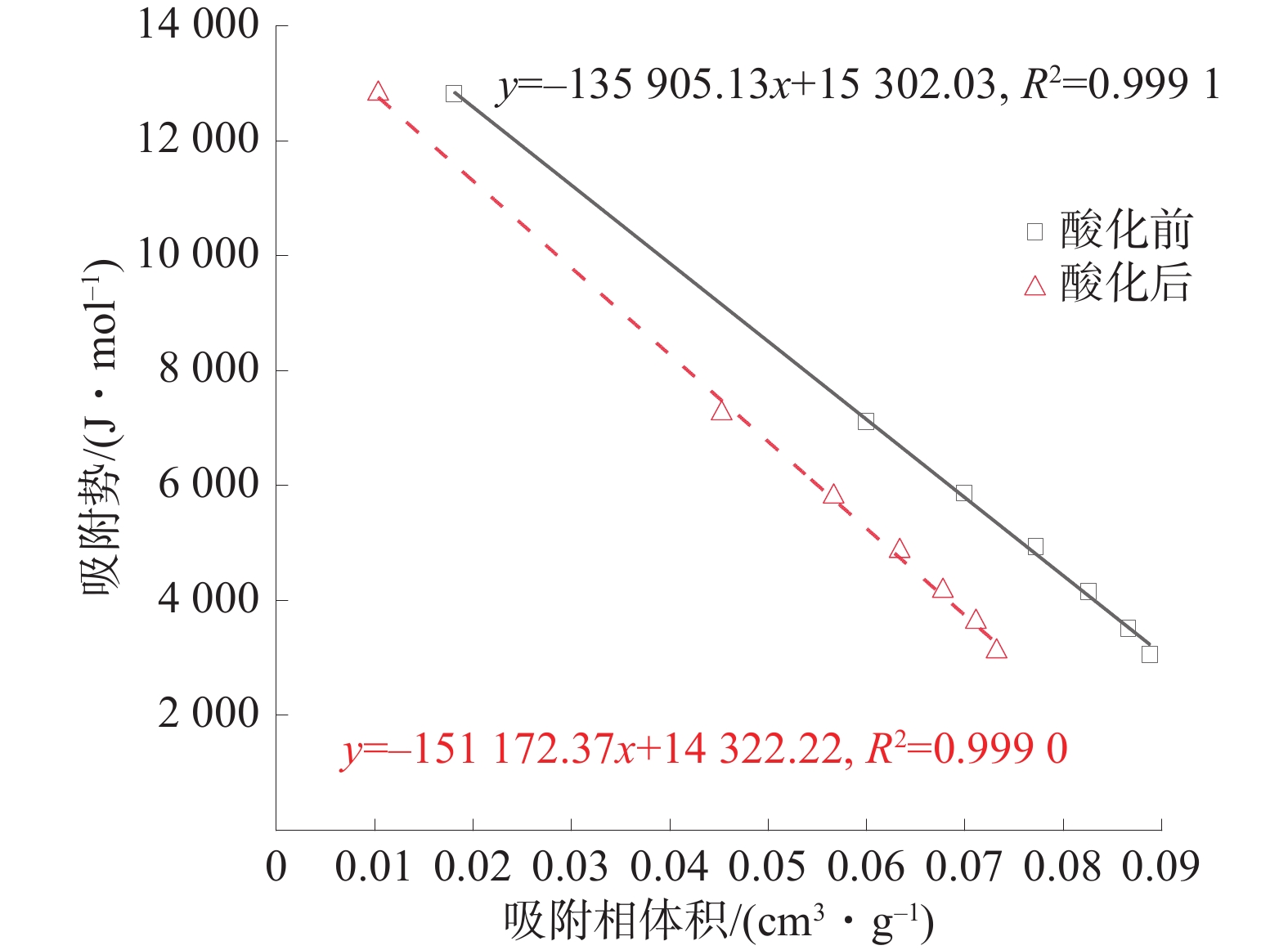
将等温吸附实验的实验数据代入式(2)~式(5),可得到吸附势和吸附相体积。酸化前后煤样的吸附势特性曲线如图6。
由图6可知,吸附等体积甲烷时,酸化后煤样的吸附势小于酸化前煤样的对应值,说明酸化后煤样对甲烷的吸附能力降低。同时,取拟合公式的截距来表征最大吸附势和最大吸附相体积,可得酸化前最大吸附势和最大吸附相体积为1.53×104 J/mol和11.26×10−2 cm3/g,而酸化后煤样的对应值分别为1.43×104 J/mol和9.47×10−2 cm3/g,下降幅度分别为6.40%和15.86%,说明酸化后煤样对甲烷的吸附能力降低、甲烷吸附空间减少。煤中甲烷的主要吸附场所为微、小孔,酸处理后煤样的平均孔径及最可几孔径增大,说明酸化后部分微、小孔的孔径增大,印证了FTIR实验中官能团反应会导致扩孔的结论。
经酸处理后,煤样对甲烷的吸附能力降低,这主要是由酸化作用对煤样微观结构的改造所致。酸化过程中,煤中活跃的有机组分及矿物质积极参与反应,煤样的表面性质及孔隙结构发生改变,煤样的亲甲烷能力下降、甲烷的吸附空间减少,进而表现为酸化后煤样对甲烷吸附能力降低。
3. 结 论
1)酸化处理后,煤中的矿物被溶蚀,平均孔径及总孔体积增大、比表面积降低、甲烷的吸附空间减小,酸液对煤体孔隙具有明显的扩孔增容作用。
2)酸处理后,苯环结构逐渐向多取代苯环发展、部分氢键结构及脂肪侧链被破坏、含氧官能团的含量上升。
3)酸处理后,煤样的吸附常数a、b值分别下降了14.02%和23.58%,说明酸化后煤样的对甲烷的极限吸附量和吸附速率下降,酸处理能够抑制煤对甲烷的吸附作用。
4)吸附势理论表明:吸附等体积甲烷时,酸化后煤样的吸附势小于酸化前煤样的对应值,且酸化作用下实验煤样的极限吸附势和吸附相体积分别下降6.40%和15.86%,说明酸化后煤样亲甲烷能力降低、甲烷的吸附空间减少,印证了酸化作用下煤中官能团反应会导致微、小孔体积增大的结论。
-
表 1 酸化前后煤样孔隙结构特征
Table 1 Pore structure characteristics of coal samples before and after acidfication
煤样 平均孔径/
nm比表面积/
(m2·g−1)孔体积/
(cm3·g−1)最可几孔径/
nm酸化前 2.64 45.0614 0.0026 1.48 酸化后 8.97 8.4283 0.0096 28.09 表 2 酸化前后煤样脂肪结构分峰拟合结果
Table 2 Peak fitting results of coal sample fat structure before and after acidification
煤样 波数/cm−1 归属 面积占比/% 酸化前 2826.46 -CH2对称振动 1.26 2853.83 -CH2对称振动 20.19 2878.85 -CH3对称振动 8.86 2900.12 -CH不对称振动 12.85 2921.84 -CH2不对称振动 34.53 2937.38 -CH3不对称振动 10.24 2959.58 -CH3不对称振动 12.06 酸化后 2827.13 -CH2对称振动 3.08 2853.20 -CH2对称振动 20.27 2877.47 -CH3对称振动 11.24 2899.00 -CH不对称振动 15.47 2921.01 -CH2不对称振动 30.05 2935.85 -CH3不对称振动 9.68 2958.18 -CH3不对称振动 10.21 表 3 酸化前后煤样羟基结构分峰拟合结果
Table 3 Fitting results of hydroxyl structure peaks in coal samples before and after acidification
煤样 波数/cm−1 归属 面积占比/% 酸化前 3197.32 环氢键 49.68 3320.74 羟基醚氢键 21.27 3439.68 自缔合羟基氢键 19.27 3540.19 羟基$ \mathrm{\pi } $氢键 9.78 酸化后 3179.24 环氢键 36.40 3414.54 自缔合羟基氢键 53.55 3512.85 羟基π氢键 10.05 -
[1] 贾男. 基于低温氮吸附法的酸化煤样孔隙分形特征研究[J]. 煤矿安全,2021,52(1):53−57. JIANan. Study on pore fractal characteristics of acidified coal samples based on low temperature nitrogen experiment[J]. Safety in Coal Mines, 2021, 52(1): 53−57.
[2] 孟召平,刘珊珊,王保玉,等. 不同煤体结构煤的吸附性能及其孔隙结构特征[J]. 煤炭学报,2015,40(8):1865−1870. MENG Zhaoping, LIU Shanshan, WANG Baoyu, et al. Adsorption capacity and its pore structure of coals with different coal body structures[J]. Journal of China Coal Society, 2015, 40(8): 1865−1870.
[3] 苏现波,汤友谊,盛建海. 河南省煤层气开发工艺初探[J]. 焦作工学院学报,1998(6):406−408. SU Xianbo, TANG Youyi, SHENG Jianhai. On three proposals of the coalbed methane development in Henan rovince[J]. Journal of Jiaozuo Institute of Technology, 1998(6): 406−408.
[4] WANG C, GAO J, ZHANG X. Effect of mixed acid fluid on the pore structure of high rank coal and acid fluid optimization[J]. ACS omega, 2022, 37(7): 33280−33294.
[5] 马永元. 表面酸液改性作用下的煤体瓦斯吸附特性[J]. 矿业安全与环保,2018,45(6):25−28. MA Yongyuan. Gas adsorption characteristics of coal affected by surface acid modification[J]. Mining Safety & Environmental Protection, 2018, 45(6): 25−28.
[6] 原文杰. 酸液改性煤样吸附性能及分形特征研究[J]. 煤矿安全,2022,53(11):31−35. YUAN Wenjie. Study on adsorption property and fractal characteristic of acid modified coal samples[J]. Safety in Coal Mines, 2022, 53(11): 31−35.
[7] 王芳芳,张小东,平晓朵,等. 酸化预处理对焦煤可溶有机质组成和结构的影响[J]. 光谱学与光谱分析,2022,42(3):896−903. doi: 10.3964/j.issn.1000-0593(2022)03-0896-08 WANG Fangfang, ZHANG Xiaodong, PING Xiaoduo, et al. Effect of acidification pretreatment on the composition andstructure of soluble organic matter in coking coal[J]. Spectroscopy and Spectral Analysis, 2022, 42(3): 896−903. doi: 10.3964/j.issn.1000-0593(2022)03-0896-08
[8] 贺成杰,杜美利,刘雷,等. 酸洗脱灰对抚顺琥珀煤结构的影响[J]. 应用化工,2018,47(12):2609−2612. HE Chengjie, DU Meili, LIU Lei, et al. Effect of demineralization on structure of Fushun amber coal[J]. Applied Chemical Industry, 2018, 47(12): 2609−2612.
[9] 张锐,袁梅,谢红飞,等. 酸化作用对煤润湿性影响试验研究[J]. 矿业研究与开发,2022,42(5):161−166. ZHANG Rui, YUAN Mei, XIE Hongfei, et al. Experimental study on the effect of acidification on coal wettability[J]. Mining Research and Development, 2022, 42(5): 161−166.
[10] 张小东,余坤坤,张硕,等. 不同类型酸作用下构造煤表面性的变化机理[J]. 煤炭转化,2017,40(3):1−7. ZHANG Xiaodong, YU Kunkun, ZHANG Shuo, et al. Change mechanism in surface properties of treated tectonic coal by different acids[J]. Coal Conversion, 2017, 40(3): 1−7.
[11] 宋申,王俊峰,王涌宇,等. 水分对褐煤微观特性的影响研究[J]. 煤炭技术,2017,36(11):330−333. SONG Shen, WANG Junfeng, WANG Yongyu, et al. Study on effect of moisture on micro-characteristics of lignite[J]. Coal Technology, 2017, 36(11): 330−333.
[12] 戚灵灵,周晓庆,彭信山,等. 基于低温氮吸附和压汞法的焦煤孔隙结构研究[J]. 煤矿安全,2022,53(7):1−6. QI Lingling, ZHOU Xiaoqing, PENG Xinshan, et al. Study on the pore structure of coking coal based on low-temperature nitrogen adsorption and mercury intrusion method[J]. Safety in Coal Mines, 2022, 53(7): 1−6.
[13] 降文萍. 煤阶对煤吸附能力影响的微观机理研究[J]. 中国煤层气,2009,6(2):19−22. JIANG Wenping. Microscopic mechanism study on the influence of coal rank on adsorption capacity[J]. China Coalbed Methane, 2009, 6(2): 19−22.
[14] 金龙哲,赵金丹,王辉,等. 煤基活性炭改性及其甲烷吸附能力[J]. 工程科学学报,2022,44(4):526−533. JIN Longzhe, ZHAO Jindan, WANG Hui, et al. Characteristic modification of coal-based activated carbon and its methane adsorption capacity[J]. Chinese Journal of Engineering, 2022, 44(4): 526−533.
[15] 梁虎珍,王传格,曾凡桂,等. 应用红外光谱研究脱灰对伊敏褐煤结构的影响[J]. 燃料化学学报,2014,42(2):129−137. LIANG Huzhen, WANG Chuange, ZENG Fangui, et al. Effect of demineralization on lignite structure from Yimin coalfield by FT-IR investufation[J]. Journal of Fuel Chemistry and Technology, 2014, 42(2): 129−137.
[16] 王宝俊,章丽娜,凌丽霞,等. 煤分子结构对煤层气吸附与扩散行为的影响[J]. 化工学报,2016,67(6):2548−2557. WANG Baojun, ZHANG Lina, LING Lixia, et al. Effect of coal molecular structure on adsorption and diffusion behaviors of coalbed methane[J]. Journal of Chemical Industry, 2016, 67(6): 2548−2557.
[17] ZHANG R, YUAN M, Li B, et al. Effects of acidification on the wettability modification of coal and absorption characteristics of coalbed methane[J]. Natural Resource Research, 2023, 32(1): 341−355. doi: 10.1007/s11053-022-10141-9
[18] XING M, XU C, ZHOU G, et al. Experimental investigation for effect of multicomponent inorganic-organic acid solution on pore structure of lignite[J]. Powder Technology, 2021, 392: 503−513. doi: 10.1016/j.powtec.2021.07.014
[19] 冯艳艳,储伟,孙文晶. 储层温度下甲烷的吸附特征[J]. 煤炭学报,2012,37(9):1488−1492. FENG Yanyan, CHU Wei, SUN Wenjing. Adsorption characteristics of methane on coal under reservoir temperatures[J]. Journal of China Coal Society, 2012, 37(9): 1488−1492.
[20] 王延斌,陶传奇,倪小明,等. 基于吸附势理论的深部煤储层吸附气量研究[J]. 煤炭学报,2018,43(6):1547−1552. WANG Yanbin, TAO Chuanqi, NI Xiaoming, et al. Amount of adsorbed gas in deep coal reservoir based on adsorption potential theory[J]. Journal of China Coal Society, 2018, 43(6): 1547−1552.
[21] 梁运培,刘盛东,魏进涛,等. 吸附势理论中饱和蒸汽压参数k探讨[J]. 煤矿安全,2016,47(12):145−148. LIANGYunpei, LIUShengdong, WEI Jintao, et al. Study on pore fractal characteristics of acidified coal samples based on low temperature nitrogen experiment[J]. Safety in Coal Mines, 2016, 47(12): 145−148.
[22] 姜伟,吴财芳,姜玮,等. 吸附势理论在煤层气吸附解吸研究中的应用[J]. 煤炭科学技术,2011,39(5):102−104. JIANG Wei, WU Caifang, JIANG Wei, et al. Application of adsorption potential theory to study on adsorption-desorption of coalbed methane[J]. Coal Science and Technology, 2011, 39(5): 102−104.
[23] 苏现波,陈润,林晓英,等. 吸附势理论在煤层气吸附/解吸中的应用[J]. 地质学报,2008(10):1382−1389. SU Xianbo, CHEN Run, LIN Xiaoying, et al. Application of adsorption potential theory in the fractionation of coalbed gas during the process of adsorption/desorption[J]. Acta Geologica Sinica, 2008(10): 1382−1389.




 下载:
下载: