Effect of acidification on micro structure and adsorption characteristics of coal
-
摘要:
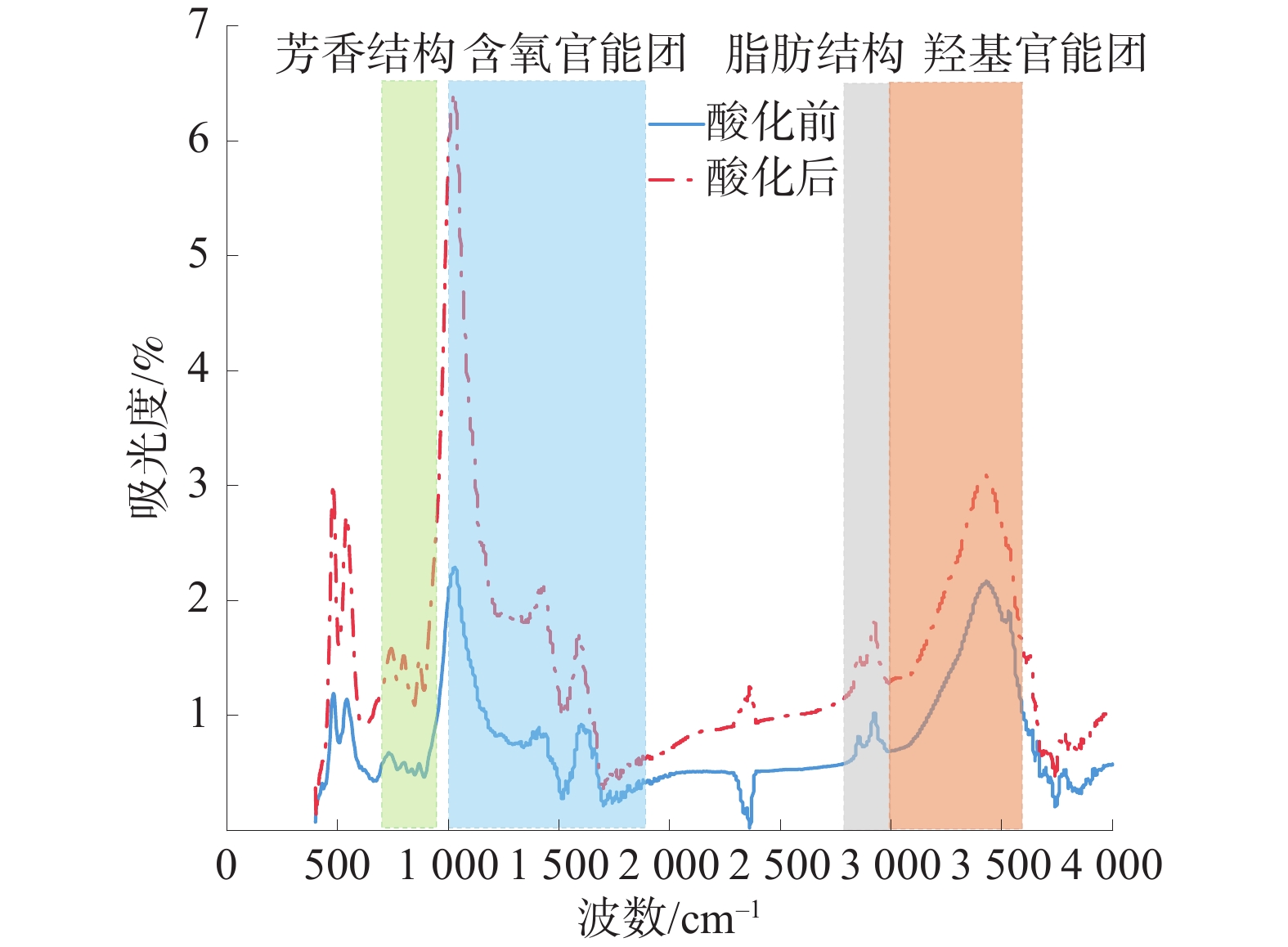
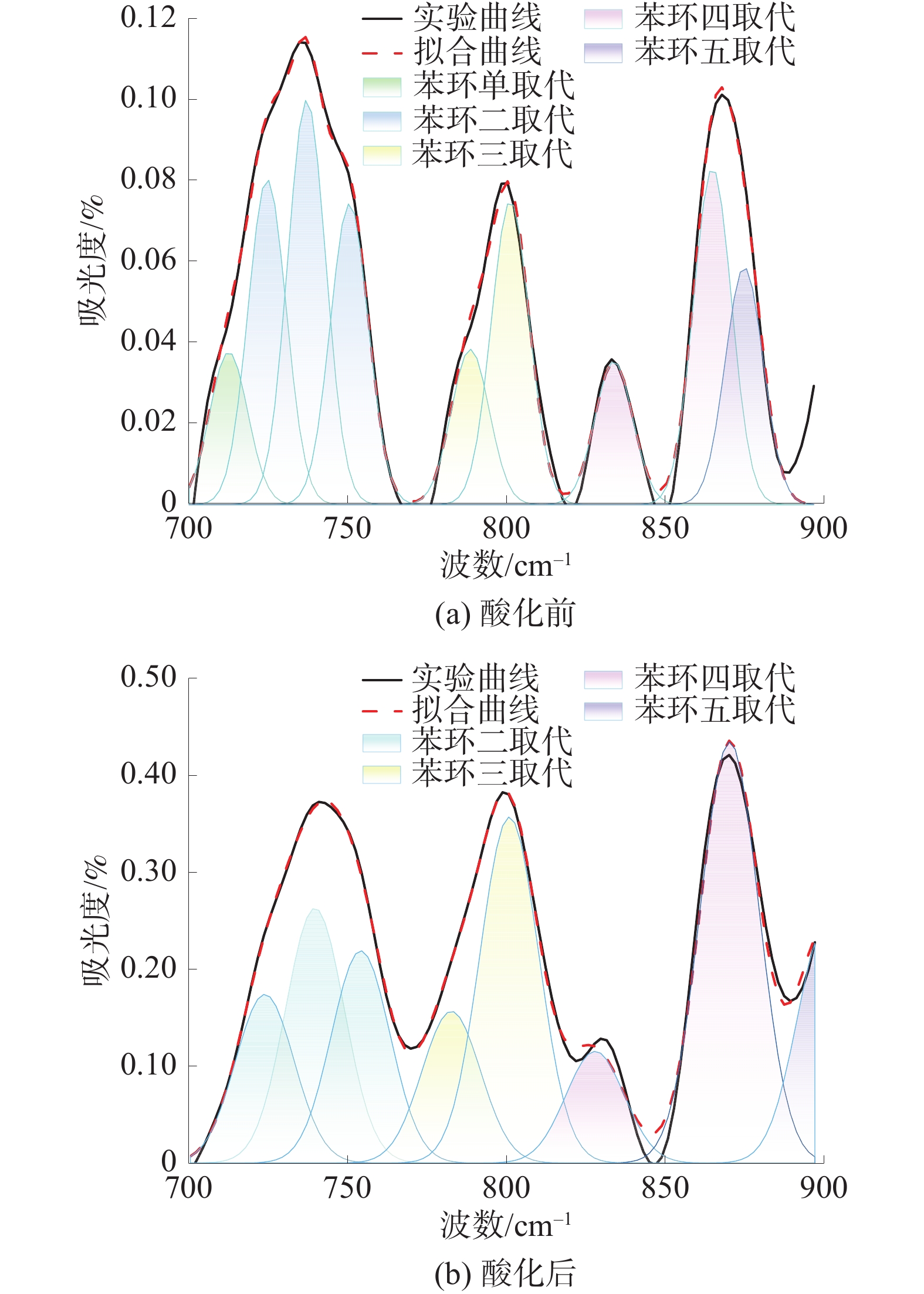
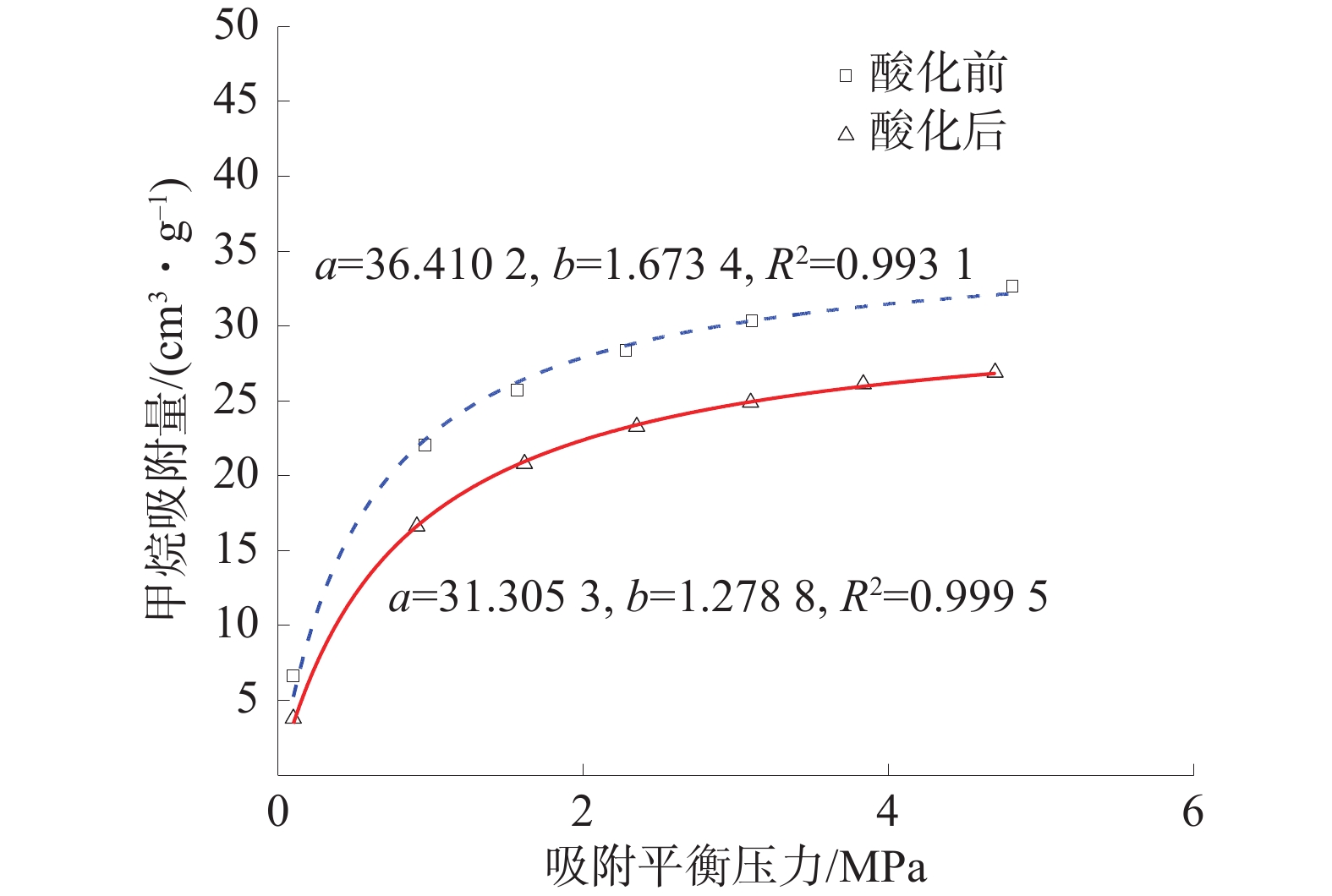
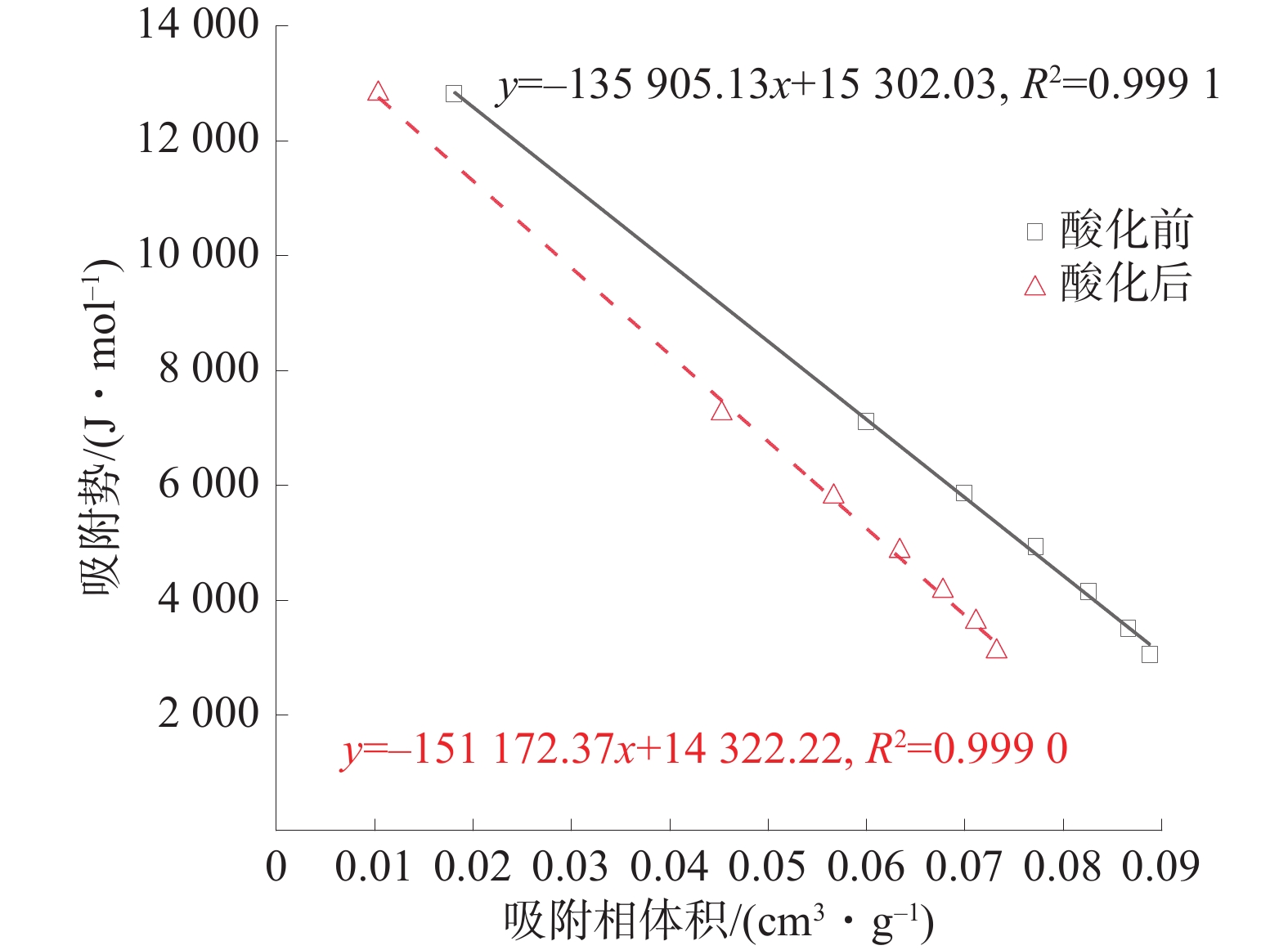
为探究酸化作用下煤体微观结构的改变及其对煤吸附特性的影响机理,对氢氟酸处理前后的煤样开展了低温氮气吸附实验、红外光谱实验及等温吸附实验,分析了酸液对煤体孔隙结构及分子结构的影响,并探究了酸化作用下煤体吸附特性的演变机制。结果表明:酸处理后,煤样的平均孔径、总孔体积增大,而比表面积降低,酸液对煤体具有扩孔增容的作用;酸化作用可以改变煤体的微观分子结构,酸化后煤样的苯环结构逐渐向多取代苯环发展,较长的脂肪链及结构较弱的氢键结构被破坏,同时含氧官能团的相对含量大幅增加;等温吸附实验中,酸化后煤样对甲烷的极限吸附量和吸附速率分别下降14.02%和23.58%;吸附势理论表明酸化后煤样对甲烷吸附能力降低、甲烷的吸附空间减少。
Abstract:To investigate the changes in the microstructure of coal under acidification and the mechanism of their impact on coal adsorption characteristics, low-temperature nitrogen adsorption experiments, infrared spectroscopy experiments, and isothermal adsorption experiments were conducted on coal samples before and after hydrofluoric acid treatment. The influence of acid solution on the pore structure and molecular structure of coal was analyzed, and the evolution mechanism of coal adsorption characteristics under acidification was explored based on the experimental results. The results showed that after acid treatment, the average pore size and total pore volume of the coal sample increased, while the specific surface area decreased. The acid solution has the effect of expanding pores and increasing capacity of pores. Acidification can change the micro molecularstructure of coal. After acidification, the benzene ring structure of coal samples gradually developed towards multi substituted benzene rings, and the longer fatty chains and weaker hydrogen bond structures were destroyed. Besides, the content of oxygen-containing functional groups increased. In the isothermal adsorption experiment, the maximum adsorption capacity and adsorption rate of methane on coal samples decreased by 14.02% and 23.58% respectively after acidification. Furthermore,the adsorption potential theory indicated that the adsorption capacity of coal samples for methane decreased and the adsorption space for methane decreases after acidification.
-
近些年来,两淮矿区煤炭生产矿井浅部资源渐趋枯竭,为确保矿井安全、高效、可持续发展,多数矿井迫切需要实施安全改建工程,在工业广场内新建深立井井筒[1]。该类工程具有井筒直径大和深度近千米等特点。由井筒检查孔勘察资料可知,这些深立井在下部基岩段施工过程中需穿过突出煤层群[2],根据相关规范规定,需要采用钻孔群进行瓦斯抽排防突,这将导致井筒揭煤抽采工程量大,特别是要在井筒围岩中施工大量瓦斯抽排钻孔,对本身就软弱的煤岩体产生强烈扰动、破损严重,不利于井筒围岩稳定和支护工作,极易造成施工的井壁结构失稳破坏[3-5]。原潘一矿二副井和望峰岗矿一副井都在井筒穿过突出煤层群揭煤抽采处出现井壁破裂事故,严重影响着井筒建设工期和矿井安全生产。因此,开展深立井过突出煤层群新型井壁结构与工程应用研究具有十分重要的工程意义。
在深立井过突出煤层群研究方面学者们进行诸多研究。王满[6]针对淮南矿区深部揭煤过程存在安全隐患难题,对潘一东矿二副井近距离揭强突出煤层群进行优化设计,通过增加2圈减压孔和多煤层联合抽采消突措施,实现了安全的井筒揭煤施工;雷文杰等[7]为防止千米深特厚煤层立井井壁混凝土初凝期间壁后瓦斯释放对井壁的致密性和完整性产生影响,提出沿井筒四周布置钻孔并通过双抗管将其引至浇筑段以外预留排放,以确保井壁混凝土质量和强度。孙仕元等[8]针对淮南矿区深立井井筒揭露多煤层突出危险性大等问题,提出采用地面预注浆加固井筒揭煤段的煤岩体;范晓刚[9]以东大煤矿回风立井为背景,提出了在立井揭穿突出厚煤层时采用锚网喷+槽钢井圈联合支护形式通过3#煤层。由上可知,在深立井过突出煤层群时,目前对揭煤处的井壁损伤加固主要采取注浆和架设井圈等措施,并没有从根本上解决井壁强度不足难题,从而导致该处井壁破损时有发生。
通过井壁受力分析和破坏机理研究表明,在井筒穿过突出煤层群揭煤抽采处,围岩破损严重,且易于片帮失稳,井壁结构受到不均匀地压作用明显,混凝土不但承受压应力,局部还要受到拉应力和剪应力作用[10]。在这种复杂应力作用下,井壁结构中混凝土极易产生破损,严重危及井筒安全运营[11-13]。因此,为了确保突出煤层群揭煤抽采处井壁结构免遭破坏,1个有效技术途径就是寻求新型支护材料及结构。
高性能钢纤维混凝土是指在混凝土基体中掺入一定量的钢纤维后而形成的1种复合材料[14-15]。与普通混凝土相比,其抗拉、抗折强度都有很大程度的提高,并且可改善混凝土脆性和韧性差的特征,特别适用于复杂受力条件下煤矿井筒支护工程[16-18]。虽然高性能钢纤维混凝土在矿山井壁结构中已得到应用,但还少有用于瓦斯突出煤层群揭煤抽采处的井筒支护工程中。综上,为了经济合理地解决突出煤层群揭煤抽采处井筒的支护难题,开展了深立井过突出煤层群高性能钢纤维混凝土井壁与工程应用研究。
1. 高性能钢纤维混凝土配制试验
根据井壁结构设计理论,煤矿深立井过瓦斯突出煤层群揭煤抽采处井壁宜采用强度等级为CF60的混凝土。为此,基于就地取材原则,首先进行配合比试验研究。
1.1 试验原材料及配合比
选用凤台海螺水泥厂生产的P. O 42.5普通硅酸盐水泥。细骨料选用天然河砂,其细度模数为2.9;粗骨料选用玄武岩碎石,其粒径为10~25 mm。
复合掺合料选用安徽美亚高新材料股份有限公司生产的NF-F,其主要成分有高效减水剂、磨细矿渣粉、粉煤灰等。
选用致泰钢纤维制造有限公司生产的成排型钢纤维,钢纤维技术参数见表1。
表 1 钢纤维技术参数Table 1. Technical parameters of steel fiber长度/mm 等效直径/mm 长径比 密度/(kg·m−3) 抗拉强度/MPa 50 0.75 67 7800 ≥ 1100 首先,通过前期正交试验,获得了C60的基准配合比(HPC-0组),然后,在此基础上掺加3种比例的钢纤维,钢纤维掺量(体积分数)分别为0.2%、0.4%、0.6%,其对应掺入质量约为15、30、45 kg/m3。各组试件编号及配合比用料结果见表2。
表 2 试验混凝土配合比Table 2. Test concrete mix ratioskg/m3 试验组号 水泥 复合掺合料 砂 石子 水 钢纤维 HPC-0 410 120 656.5 1105.4 145.8 0 HPC-0.2 410 120 656.5 1105.4 145.8 15 HPC-0.4 410 120 656.5 1105.4 145.8 30 HPC-0.6 410 120 656.5 1105.4 145.8 45 1.2 试件制备与试验方法
根据以上配合比,针对不同的试验内容分别制作混凝土试件。试验采用对比方法进行,其中1组为基准配合比试件(HPC-0),其它3组分别为3个不同体积掺量的钢纤维混凝土试件组。其中:进行抗压强度和抗拉强度试验的试件尺寸为100 mm$ \times $100 mm$ \times $100 mm,抗弯强度试验的试件尺寸为100 mm $ \times $100 mm$ \times $515 mm,它们均为非标准尺寸试件,试验结果需要按照规程换算成标准尺寸值。
试件浇筑成型后,立即用不透水的薄膜覆盖试件表面,然后在标准养护环境(温度(20±2) ℃,湿度95%以上)中放置24 h,再脱模放入YH-40标准养护箱中养护。待试件养护达到28 d龄期后,将其从养护箱取出,先用干布擦拭干净,再按照试验规程要求放置于压力试验机中。
1)抗压强度试验。抗压强度试验控制加载速率0.2~0.3 MPa/s,当试件接近破坏并开始急剧变形时,停止调整试验机油门,直至试件破坏。抗压强度试验加载示意图及破坏形态如图1所示。
2)劈裂抗拉强度试验。劈裂抗拉强度试验控制加载速率为0.08~0.10 MPa/s,在试件的上、下端面处使用半圆形钢垫条,将试件中心线与垫块轴心对准后,开始试验。劈裂抗拉强度试验加载示意图及破坏形态如图2所示。
3)抗折强度试验。抗折强度试验控制加载速率0.08~0.10 MPa/s,使用抗折试验装置,可使2个相等荷载同时作用在试件跨度3分点处。抗折强度试验加载示意图及破坏形态如图3所示。
1.3 力学性能试验结果及其分析
对试件进行抗压、抗拉、抗折强度试验。抗压强度试验结果见表3,抗拉强度试验结果见表4,抗折强度试验结果见表5,抗折试验加载曲线如图4所示。
表 3 抗压强度试验结果Table 3. Compressive strength test results试件 HPC-0 HPC-0.2 HPC-0.4 HPC-0.6 抗压强度/MPa 69.9 72.1 73.6 73.9 提升幅度/% 0 3.1 5.3 5.7 表 4 抗拉强度试验结果Table 4. Tensile strength test results试件 HPC-0 HPC-0.2 HPC-0.4 HPC-0.6 抗拉强度/MPa 4.8 5.9 6.6 6.7 提升幅度/% 0 22.9 37.5 39.6 表 5 抗折强度试验结果Table 5. Flexural strength test results试件 HPC-0 HPC-0.2 HPC-0.4 HPC-0.6 抗折强度/MPa 6.1 7.9 9.3 9.8 提升幅度/% 0 29.5 52.5 60.7 由表3可见:3种钢纤维掺量下高性能钢纤维混凝土抗压强度值相差不大,相比基准混凝土虽然有一定程度提高,但最大幅度仅为5.7%。由此可见,增加钢纤维含量对井壁混凝土抗压强度的提升效果并不明显,尤其是在钢纤维掺量达到0.4%以后。
由表4可见:与未添加钢纤维的基准组相比,钢纤维对混凝土基体的抗拉性能提升效果十分显著;当钢纤维掺量为0.4%时,提高幅度达到37.5%;而当钢纤维掺量达到0.6%时,提高幅度为39.6%,抗拉强度增加有限,但工程成本明显增加。同时,钢纤维掺量大时,也会导致混凝土内部纤维分散均匀性变差,影响混凝土整体浇注质量[19]。
由表5可见:不同钢纤维掺量下的钢纤维混凝土抗折强度较基准混凝土提高效果显著,当掺量为0.4%时提高幅度达到52.5%;而当掺量为0.6%时,其抗折强度进一步提高但幅度有限。
由图4可见:基准组混凝土在达到峰值后,应力下降迅速,在短时间内完全破坏,脆性破坏现象非常明显;掺加钢纤维后的高性能混凝土峰值应力明显高于未掺加纤维基准混凝土,而且承受荷载的时间更长;在应力降低至约2 MPa时,抗折应力趋于平缓,表现出良好的塑性特征。从破坏试件的断口处也可明显观察到,一部分钢纤维被拔出,一部分被拉断,表明钢纤维在混凝土开裂后,开始抵抗裂缝的扩展。随着钢纤维掺量的增加,抗折曲线随时间的延伸长度也随之增加,但当钢纤维掺量达到0.4%以后,曲线延伸有限。
综合对比4组混凝土的抗压、抗拉、抗折强度试验结果可以看出:在考虑增强效果和工程造价后,当钢纤维体积掺量为0.4%时,即HPC-0.4组的综合性能指标要优于其他试验组。因此,试验最终确定每立方米高性能钢纤维混凝土的最优配合比为水泥∶复合掺合料∶砂∶石子∶水∶钢纤维= 410∶120∶656.5∶
1105.4 ∶145.8∶30,材料用量单位为kg。2. 井壁结构模拟试验
2.1 工程概况
丁集煤矿第二回风井筒设计净直径7.5 m,上部冲积层和风化基岩段采用冻结法凿井,冻结深度574 m,下部基岩段449 m采用地面预注浆止水、钻爆法施工。该井筒在下部基岩段施工时将要穿过10多层突出煤层,根据相关规范要求,需进行揭煤防突。煤层赋存分布见表6。
表 6 煤层赋存分布Table 6. Coal seam distribution table煤层 厚度/m 顶板 底板 备注 4-2 2.1 细粒砂岩 细粒砂岩 无 4-1 5.1 细粒砂岩 砂质泥岩 与4-2煤间距4.1 m 煤线5 1.1 泥岩 砂质泥岩 与4-1煤间距4.0 m 勘探资料显示,4-2煤上部以块状为主,下部为碎片状、粉末状;4-1煤为碎块状、粉末状,夹矸为泥岩;煤线5为碎片状、粉末状为主,少许块状,并且在揭煤段井筒附近发育有逆断层F151-4-2(高H=3.0 m),对掘进和围岩稳定有一定影响。由此可见,该处煤岩软弱,自身稳定性差。
当井筒工作面掘砌至距4-2煤顶板10 m位置停头,首先施工4个前探孔,然后施工4个测压孔;根据相关探测和测压结果,再对4-2煤、4-1煤、煤线5施工区域防突措施钻孔。根据有关规定设计布置12圈、共计412个抽采孔,钻孔量为13 592.6 m。钻孔控制到揭煤处井筒荒径轮廓线外不小于15 m,且钻孔控制范围的外边缘到井筒荒径轮廓线的最小距离不小于7 m。钻孔按照有效抽采半径不大于1.5 m设计,开孔间距0.5 m,1-9圈孔穿过4-2煤、4-1煤、煤线5并进入煤线5底板不小于5 m,第10圈孔穿过4-2煤、4-1煤并进入4-1煤底板不小于5 m,第11、第12圈孔穿过4-2煤并进入4-2煤底板不小于5 m。根据原设计,揭煤段采用700 mm厚素混凝土井壁,混凝土强度等级为C60。
由于该井筒揭煤处施工了400多个瓦斯抽采防突钻孔,大量密集的钻孔造成井筒周边原本破碎、软弱的煤岩体更加破碎,不但井筒掘进时易发生围岩失稳,而且还极易造成井壁破坏,影响井筒的运营安全。因此,需开展井筒揭煤处CF60新型井壁结构研究。
2.2 井壁结构模型设计和加工
井壁结构模拟试验以丁集煤矿第二回风井筒揭4-2煤、4-1煤、煤线5区域防突处井筒支护为工程原型,开展试验研究。由于原型混凝土井壁结构几何尺寸大,不易进行室内破坏性试验,本试验采用缩尺井壁结构模型。
本次模型试验不仅要获得加载过程中井壁截面上的应力分布情况,而且还要测量井壁模型的破坏荷载。所以,井壁模型设计不但要满足应力、变形相似条件,而且还要满足强度相似条件。
根据模型试验相似理论[20],为了易于满足相似准则、确保模型井壁的受力状态与原型井壁一致,模型试验井壁试件宜采用原井壁结构材料,即采用CF60高性能钢纤维混凝土,则有:
$$\begin{split}& \begin{gathered} {C_{{r}}} = {C_{{u}}} \\ {C_{{E}}} = {C_\sigma } = {C_{{X}}} = {C_{{R}}} = {C_{\varepsilon}} = {C_{\xi}} = 1 \end{gathered}\\[-16pt]& \end{split} $$ (1) 式中:${C_{\varepsilon}}$为应变相似常数;${C_{{r}}}$为几何相似常数;${C_{{u}}}$为位移相似常数;${C_{{X}}}$为荷载(面力)相似常数;${C_\sigma }$为应力相似常数;${C_{{E}}}$为弹性模量相似常数;$ {C_{{R}}} $为强度相似常数;$ {C_{\xi}} $为钢纤维体积率相似常数。
查阅丁集煤矿第二回风井筒的工程概况,原型井壁设计参数为:井壁外径8.9 m,井壁内径7.5 m,壁厚700 mm,混凝土设计强度CF60。
由于第二回风立井揭煤段井筒附近发育有逆断层,又施工大量瓦斯抽采钻孔,井壁受力条件十分复杂。为此,试验采用不均匀荷载模式。首先,基于配制试验和力学性能测试结果,以钢纤维体积掺量为0.4%的配合比进行井壁模型的浇筑,对CF60钢纤维混凝土井壁进行加载试验,以获得其主要力学特性。同时,为了对比试验结果,又对原设计C60素混凝土井壁进行试验。每种井壁结构制作2个模型试件,共进行4次模型试验,模型几何相似比为2.966。各井壁模型参数见表7。
表 7 井壁模型设计参数Table 7. Design parameters of shaft lining model模型
编号外直径/
mm内直径/
mm壁厚/
mm高度/
mm钢纤维掺量/
(kg·m−3)混凝土
设计强度CF1 3 000 2528 236 210 30 CF60 CF2 3 000 2528 236 210 30 CF60 C1 3 000 2528 236 210 0 C60 C2 3 000 2528 236 210 0 C60 模型试件的浇注采用专门加工的模具,立好模板后进行混凝土浇筑,待试件养护到龄期前,对上端面进行打磨,以确保端面平整。浇筑完成的井壁模型试件如图5所示。
2.3 试验和测试方法
模型加载试验在大型井壁结构试验台座内进行,采用16台2 000 kN高压油缸来模拟井壁承受的水平荷载,竖向通过地脚螺栓和拉杆约束,使井壁试件处于平面受力状态,与实际工程中采用短段掘砌的井壁结构基本一致。
根据GB 50384—2016《煤矿立井井筒及硐室设计规范》 ,通常井壁荷载的不均匀压力系数按1.1~1.2计算,即大荷载方向与小荷载方向的荷载值之比为1.1~1.2倍。结合本次试验的16台2 000 kN高压油缸布置方式设计了加载方法:东、西方向8个油缸施加大荷载,南、北方向8个油缸施工小荷载,大、小荷载比值为1.18倍。通过井壁截面受力分析及等效原理可知,对井壁结构来说,这种加载方式比正弦分布的不均匀荷载作用更为不利。因此,试验施加的不均匀压力系数1.18相当于正弦函数分布形式的1.2。
为获得井壁模型在整个试验加载过程中的变形和应力变化情况,分析井壁的受力特性,在井壁模型表面粘贴了48个电阻应变计。同时,在模型内侧布置4个位移计,以观测井壁在不均匀荷载作用下的径向位移。
为获得井壁模型的受力过程和极限承载力,试验采用逐级加载法,每级荷载增加0.5 MPa,直到井壁发生破坏。在每级荷载作用下待其变形稳定后,再采集测量数据。
2.4 试验结果及分析
2.4.1 井壁截面应变特征
通过粘贴在井壁内侧表面的应变计可测得混凝土的环向应变和竖向应变,以全面分析井壁模型在不同荷载作用下的变形特性。CF1模型试件施加大、小荷载方向井壁截面应变变化情况如图6所示。
由图6可见:在大荷载作用方向,井壁截面内缘环向应变处于受拉状态;而在小荷载作用方向,则正好相反,其内缘的环向应变处于受压状态。特别是在大荷载作用方向的内缘,在加载初期就开始出现了拉应变,极易造成对比组的素混凝土井壁开裂和破坏。所以说,在复杂工程地质条件下,由于井壁受力复杂,采用钢纤维混凝土对防止井壁受拉、受剪破坏、提高井壁承载能力是十分有利的。
2.4.2 井壁结构的应力分布
模型试验中井壁结构处于平面应力状态,在弹性变形阶段,井壁的内、外缘应力分布符合弹性厚壁圆筒理论。当井壁变形由弹性进入塑性阶段后,井壁内混凝土的本构关系已不符合虎克定律。为此,引入单一曲线假设,采用计算机迭代运算,求出变形模量和泊松比,再求对应的应力值。CF1井壁模型主要部位在荷载作用级下的应力变化曲线如图7所示。
由图7可见:在大荷载作用方向,井壁截面内侧出现拉应力;在小荷载作用方向,截面内侧处于受压应力状态。特别是在大荷载作用方向,井壁内缘开始出现了拉应力,这对原设计的素混凝土井壁受力是极为不利的,导致混凝土过早开裂,降低了井壁承载能力。而采用钢纤维混凝土则可大大提高材料的抗拉和抗折强度,避免井壁过早开裂破坏,可显著提高井壁的承载力,这就是不均匀荷载作用下,高性能钢纤维混凝土井壁可提高井壁强度的机理。
2.4.3 井壁承载力
通过对模型试件逐级加载直至破坏,得到的井壁模型试件的极限承载力见表8。
表 8 不均匀荷载作用下井壁模型试验承载力Table 8. Bearing capacity of shaft lining model test under uneven loads模型编号 混凝土抗压强度/MPa 承载力/MPa 小荷载 大荷载 CF1 70.3 5.2 6.1 CF2 69.8 5.1 6.0 C1 70.2 4.5 5.3 C2 71.6 4.6 5.4 由表8可见:在不均匀荷载作用下,高性能钢纤维混凝土井壁承载力比原设计的素混凝土井壁提高了约13.2%~15.6%,这主要是在不均匀荷载作用下,模型在大荷载方向,外侧压应力集中程度加大;而在小荷载方向,则是内侧压应力集中程度加大。特别是在大荷载作用方向,井壁内缘开始出现了拉应力,导致井壁承载能力降低。而采用高性能钢纤维混凝土可大大提高材料的抗拉强度和抗折强度,显著地提高了井壁的承载能力。
2.4.4 井壁破坏形态
井壁模型破坏形态如图8所示。
由加载试验可知,井壁模型在不均匀荷载作用下,破坏位置主要位于大荷载内缘,此处混凝土受偏心压力和弯矩的作用,出现了较大拉应力。由于原设计的素混凝土井壁抗拉强度低、延性较差,破坏前没有明显的预兆,具有突然性,破坏时发出较大的响声,局部混凝土呈断裂状(图8(a))。而钢纤维混凝土井壁由于加入了钢纤维,显著提高了混凝土的抗拉和抗剪强度,改善了混凝土的脆性,使其延性大大提高,因而井壁在破坏时首先出现斜向微裂纹,并慢慢扩大,在井壁丧失极限承载能力时,破裂面仍通过钢纤维连接在一起(图8(b))。所以,在复杂条件下的井筒支护工程中,采用高性能钢纤维混凝土井壁可大大改善井壁的变形特征,提高工程结构的安全性。
3. 工程应用
通过以上力学特性及其井壁结构模型试验研究表明,高性能钢纤维混凝土由于其抗拉强度、抗折强度和抗裂性能显著提高,使得井壁结构在不均匀荷载作用下承载能力得到显著提高。为此,在丁集煤矿第二回风井筒下部揭煤处采用了该种新型井壁结构代替原先设计的素混凝土井壁结构,钢纤维体积掺量为0.4%。
当井筒掘进到4-1煤段,采用700 mm厚、CF60高性能钢纤维混凝土井壁代替原设计的C60素混凝土井壁,为研究分析该种新型井壁结构的实际工程受力特性,及时进行井壁结构的安全性评价,确保井筒施工和使用安全,沿井壁圆周分4个方向埋设了环向和竖向混凝土应变计。通过现场观测,得到的高性能钢纤维混凝土井壁环向应变随时间变化曲线如图9所示。
由图9可见:高性能钢纤维混凝土井壁在埋设测试元件的4个方向所受应变极不均匀,这主要是所处位置附近存在小断层和施工了大量瓦斯抽排钻孔引起的,虽然井壁混凝土产生了较大的应变,但由于采用了钢纤维混凝土提高了井壁的承载力,所以,目前井壁结构受力稳定。实测环向应变为(−202~−591)×10−6,过小于钢纤维混凝土极限应变值,井壁结构是安全可靠的,可确保井筒安全运营。
4. 结 语
1)力学性能试验结果表明:钢纤维掺量为0.4%的高性能钢纤维混凝土抗压强度比基准混凝土高5.3%;而抗拉强度和抗折强度比基准混凝土分别高37.5%和52.5%,提升幅度相比抗压强度更加显著。
2)井壁结构模拟试验结果表明:在不均匀荷载作用下,钢纤维掺量为0.4%的高性能钢纤维混凝土井壁承载力比原设计的素混凝土井壁提高了约13.2%~15.6%;且在井壁结构中加入钢纤维后,改善了混凝土的脆性、使得延性大大提高,在井壁丧失极限承载能力时,破裂面仍通过钢纤维连接在一起,提高井壁的安全性。
3)通过新型井壁结构的工程应用和现场实测结果表明:目前井壁结构受力稳定,实测环向应变为(−230.0~−587.2)×10−6,远小于钢纤维混凝土极限应变值,井壁结构是安全可靠的,可确保井筒安全运营,可在类似条件下推广应用。
-
表 1 酸化前后煤样孔隙结构特征
Table 1 Pore structure characteristics of coal samples before and after acidfication
煤样 平均孔径/
nm比表面积/
(m2·g−1)孔体积/
(cm3·g−1)最可几孔径/
nm酸化前 2.64 45.0614 0.0026 1.48 酸化后 8.97 8.4283 0.0096 28.09 表 2 酸化前后煤样脂肪结构分峰拟合结果
Table 2 Peak fitting results of coal sample fat structure before and after acidification
煤样 波数/cm−1 归属 面积占比/% 酸化前 2826.46 -CH2对称振动 1.26 2853.83 -CH2对称振动 20.19 2878.85 -CH3对称振动 8.86 2900.12 -CH不对称振动 12.85 2921.84 -CH2不对称振动 34.53 2937.38 -CH3不对称振动 10.24 2959.58 -CH3不对称振动 12.06 酸化后 2827.13 -CH2对称振动 3.08 2853.20 -CH2对称振动 20.27 2877.47 -CH3对称振动 11.24 2899.00 -CH不对称振动 15.47 2921.01 -CH2不对称振动 30.05 2935.85 -CH3不对称振动 9.68 2958.18 -CH3不对称振动 10.21 表 3 酸化前后煤样羟基结构分峰拟合结果
Table 3 Fitting results of hydroxyl structure peaks in coal samples before and after acidification
煤样 波数/cm−1 归属 面积占比/% 酸化前 3197.32 环氢键 49.68 3320.74 羟基醚氢键 21.27 3439.68 自缔合羟基氢键 19.27 3540.19 羟基$ \mathrm{\pi } $氢键 9.78 酸化后 3179.24 环氢键 36.40 3414.54 自缔合羟基氢键 53.55 3512.85 羟基π氢键 10.05 -
[1] 贾男. 基于低温氮吸附法的酸化煤样孔隙分形特征研究[J]. 煤矿安全,2021,52(1):53−57. JIANan. Study on pore fractal characteristics of acidified coal samples based on low temperature nitrogen experiment[J]. Safety in Coal Mines, 2021, 52(1): 53−57.
[2] 孟召平,刘珊珊,王保玉,等. 不同煤体结构煤的吸附性能及其孔隙结构特征[J]. 煤炭学报,2015,40(8):1865−1870. MENG Zhaoping, LIU Shanshan, WANG Baoyu, et al. Adsorption capacity and its pore structure of coals with different coal body structures[J]. Journal of China Coal Society, 2015, 40(8): 1865−1870.
[3] 苏现波,汤友谊,盛建海. 河南省煤层气开发工艺初探[J]. 焦作工学院学报,1998(6):406−408. SU Xianbo, TANG Youyi, SHENG Jianhai. On three proposals of the coalbed methane development in Henan rovince[J]. Journal of Jiaozuo Institute of Technology, 1998(6): 406−408.
[4] WANG C, GAO J, ZHANG X. Effect of mixed acid fluid on the pore structure of high rank coal and acid fluid optimization[J]. ACS omega, 2022, 37(7): 33280−33294.
[5] 马永元. 表面酸液改性作用下的煤体瓦斯吸附特性[J]. 矿业安全与环保,2018,45(6):25−28. MA Yongyuan. Gas adsorption characteristics of coal affected by surface acid modification[J]. Mining Safety & Environmental Protection, 2018, 45(6): 25−28.
[6] 原文杰. 酸液改性煤样吸附性能及分形特征研究[J]. 煤矿安全,2022,53(11):31−35. YUAN Wenjie. Study on adsorption property and fractal characteristic of acid modified coal samples[J]. Safety in Coal Mines, 2022, 53(11): 31−35.
[7] 王芳芳,张小东,平晓朵,等. 酸化预处理对焦煤可溶有机质组成和结构的影响[J]. 光谱学与光谱分析,2022,42(3):896−903. doi: 10.3964/j.issn.1000-0593(2022)03-0896-08 WANG Fangfang, ZHANG Xiaodong, PING Xiaoduo, et al. Effect of acidification pretreatment on the composition andstructure of soluble organic matter in coking coal[J]. Spectroscopy and Spectral Analysis, 2022, 42(3): 896−903. doi: 10.3964/j.issn.1000-0593(2022)03-0896-08
[8] 贺成杰,杜美利,刘雷,等. 酸洗脱灰对抚顺琥珀煤结构的影响[J]. 应用化工,2018,47(12):2609−2612. HE Chengjie, DU Meili, LIU Lei, et al. Effect of demineralization on structure of Fushun amber coal[J]. Applied Chemical Industry, 2018, 47(12): 2609−2612.
[9] 张锐,袁梅,谢红飞,等. 酸化作用对煤润湿性影响试验研究[J]. 矿业研究与开发,2022,42(5):161−166. ZHANG Rui, YUAN Mei, XIE Hongfei, et al. Experimental study on the effect of acidification on coal wettability[J]. Mining Research and Development, 2022, 42(5): 161−166.
[10] 张小东,余坤坤,张硕,等. 不同类型酸作用下构造煤表面性的变化机理[J]. 煤炭转化,2017,40(3):1−7. ZHANG Xiaodong, YU Kunkun, ZHANG Shuo, et al. Change mechanism in surface properties of treated tectonic coal by different acids[J]. Coal Conversion, 2017, 40(3): 1−7.
[11] 宋申,王俊峰,王涌宇,等. 水分对褐煤微观特性的影响研究[J]. 煤炭技术,2017,36(11):330−333. SONG Shen, WANG Junfeng, WANG Yongyu, et al. Study on effect of moisture on micro-characteristics of lignite[J]. Coal Technology, 2017, 36(11): 330−333.
[12] 戚灵灵,周晓庆,彭信山,等. 基于低温氮吸附和压汞法的焦煤孔隙结构研究[J]. 煤矿安全,2022,53(7):1−6. QI Lingling, ZHOU Xiaoqing, PENG Xinshan, et al. Study on the pore structure of coking coal based on low-temperature nitrogen adsorption and mercury intrusion method[J]. Safety in Coal Mines, 2022, 53(7): 1−6.
[13] 降文萍. 煤阶对煤吸附能力影响的微观机理研究[J]. 中国煤层气,2009,6(2):19−22. JIANG Wenping. Microscopic mechanism study on the influence of coal rank on adsorption capacity[J]. China Coalbed Methane, 2009, 6(2): 19−22.
[14] 金龙哲,赵金丹,王辉,等. 煤基活性炭改性及其甲烷吸附能力[J]. 工程科学学报,2022,44(4):526−533. JIN Longzhe, ZHAO Jindan, WANG Hui, et al. Characteristic modification of coal-based activated carbon and its methane adsorption capacity[J]. Chinese Journal of Engineering, 2022, 44(4): 526−533.
[15] 梁虎珍,王传格,曾凡桂,等. 应用红外光谱研究脱灰对伊敏褐煤结构的影响[J]. 燃料化学学报,2014,42(2):129−137. LIANG Huzhen, WANG Chuange, ZENG Fangui, et al. Effect of demineralization on lignite structure from Yimin coalfield by FT-IR investufation[J]. Journal of Fuel Chemistry and Technology, 2014, 42(2): 129−137.
[16] 王宝俊,章丽娜,凌丽霞,等. 煤分子结构对煤层气吸附与扩散行为的影响[J]. 化工学报,2016,67(6):2548−2557. WANG Baojun, ZHANG Lina, LING Lixia, et al. Effect of coal molecular structure on adsorption and diffusion behaviors of coalbed methane[J]. Journal of Chemical Industry, 2016, 67(6): 2548−2557.
[17] ZHANG R, YUAN M, Li B, et al. Effects of acidification on the wettability modification of coal and absorption characteristics of coalbed methane[J]. Natural Resource Research, 2023, 32(1): 341−355. doi: 10.1007/s11053-022-10141-9
[18] XING M, XU C, ZHOU G, et al. Experimental investigation for effect of multicomponent inorganic-organic acid solution on pore structure of lignite[J]. Powder Technology, 2021, 392: 503−513. doi: 10.1016/j.powtec.2021.07.014
[19] 冯艳艳,储伟,孙文晶. 储层温度下甲烷的吸附特征[J]. 煤炭学报,2012,37(9):1488−1492. FENG Yanyan, CHU Wei, SUN Wenjing. Adsorption characteristics of methane on coal under reservoir temperatures[J]. Journal of China Coal Society, 2012, 37(9): 1488−1492.
[20] 王延斌,陶传奇,倪小明,等. 基于吸附势理论的深部煤储层吸附气量研究[J]. 煤炭学报,2018,43(6):1547−1552. WANG Yanbin, TAO Chuanqi, NI Xiaoming, et al. Amount of adsorbed gas in deep coal reservoir based on adsorption potential theory[J]. Journal of China Coal Society, 2018, 43(6): 1547−1552.
[21] 梁运培,刘盛东,魏进涛,等. 吸附势理论中饱和蒸汽压参数k探讨[J]. 煤矿安全,2016,47(12):145−148. LIANGYunpei, LIUShengdong, WEI Jintao, et al. Study on pore fractal characteristics of acidified coal samples based on low temperature nitrogen experiment[J]. Safety in Coal Mines, 2016, 47(12): 145−148.
[22] 姜伟,吴财芳,姜玮,等. 吸附势理论在煤层气吸附解吸研究中的应用[J]. 煤炭科学技术,2011,39(5):102−104. JIANG Wei, WU Caifang, JIANG Wei, et al. Application of adsorption potential theory to study on adsorption-desorption of coalbed methane[J]. Coal Science and Technology, 2011, 39(5): 102−104.
[23] 苏现波,陈润,林晓英,等. 吸附势理论在煤层气吸附/解吸中的应用[J]. 地质学报,2008(10):1382−1389. SU Xianbo, CHEN Run, LIN Xiaoying, et al. Application of adsorption potential theory in the fractionation of coalbed gas during the process of adsorption/desorption[J]. Acta Geologica Sinica, 2008(10): 1382−1389.



 下载:
下载: